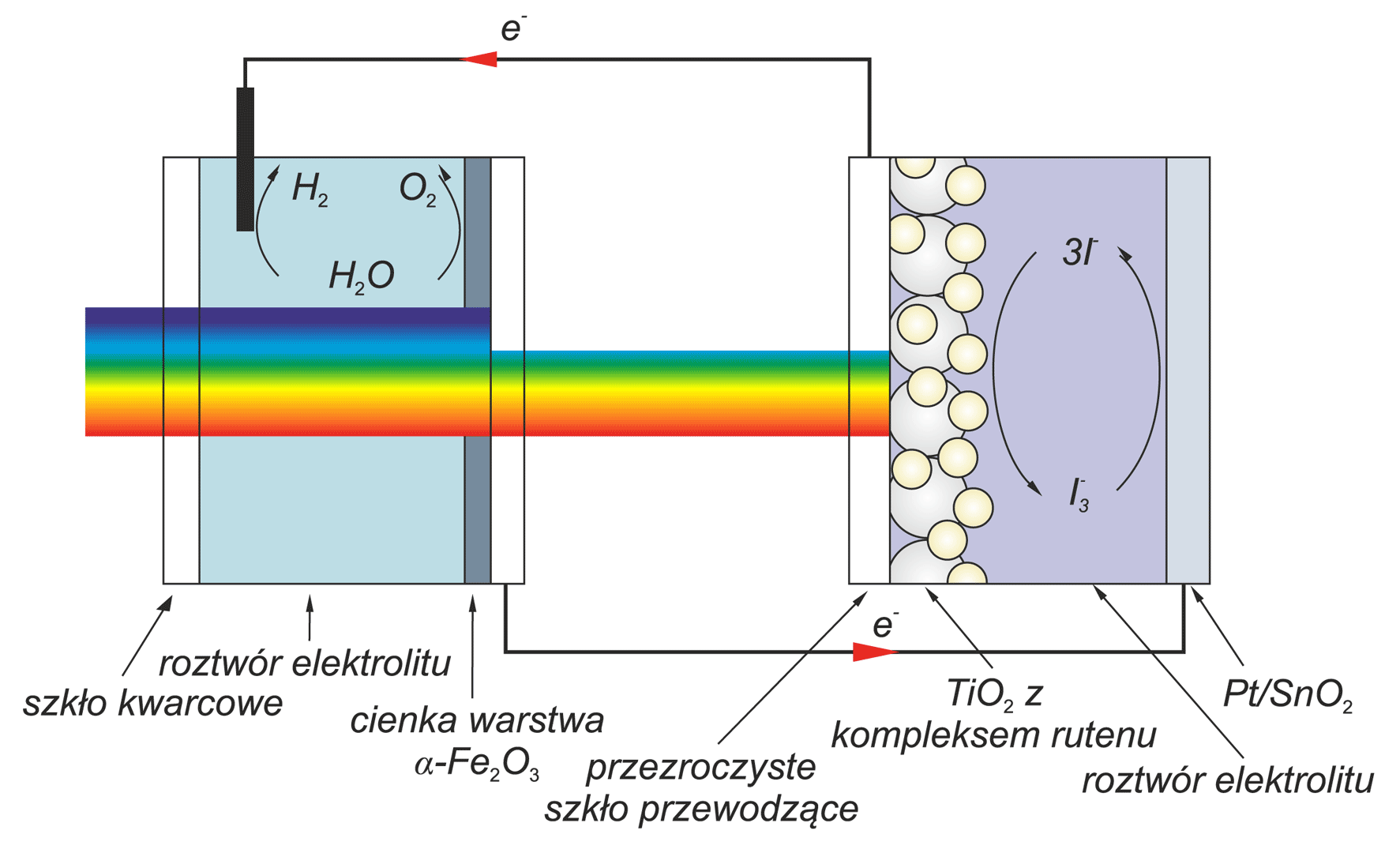
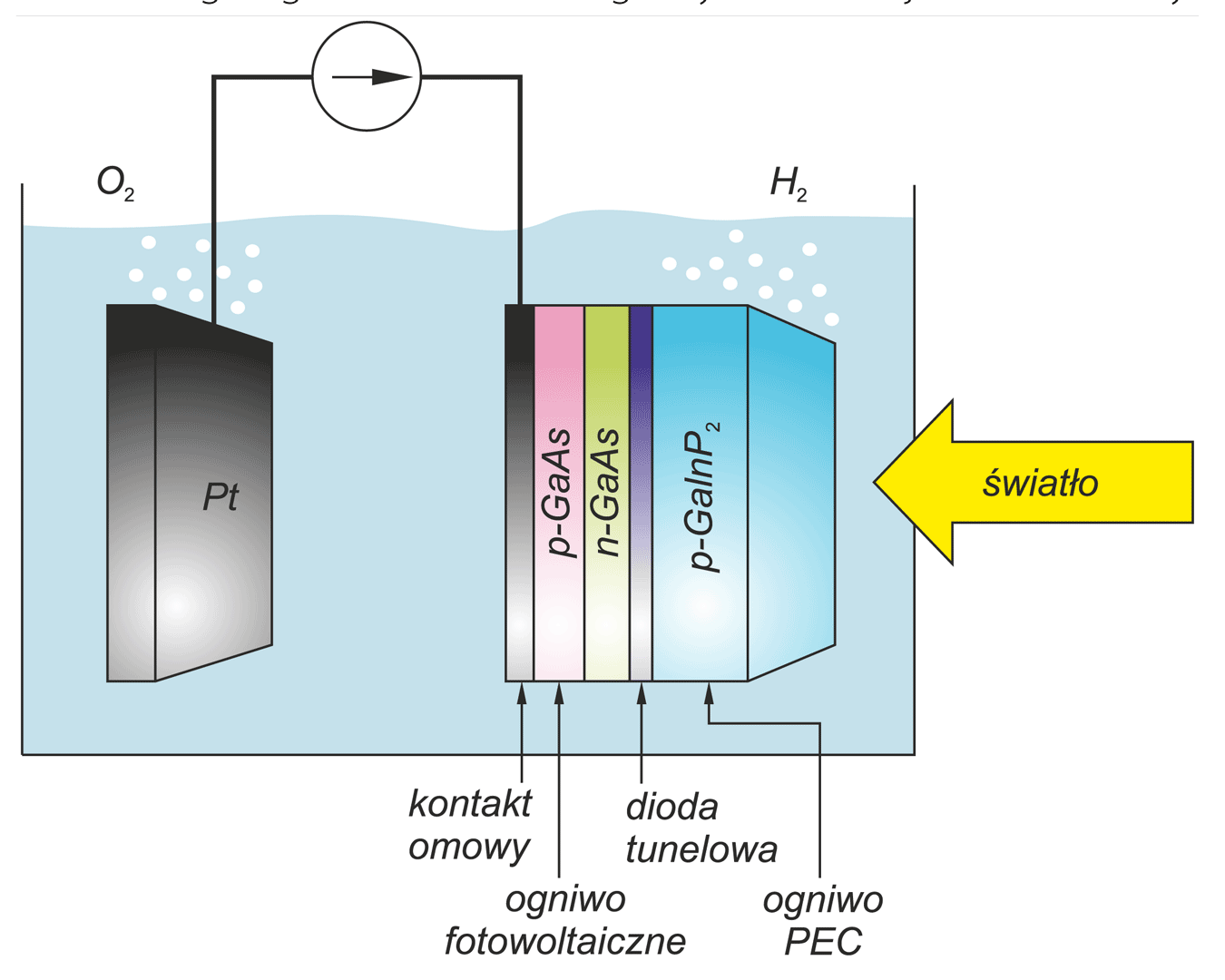
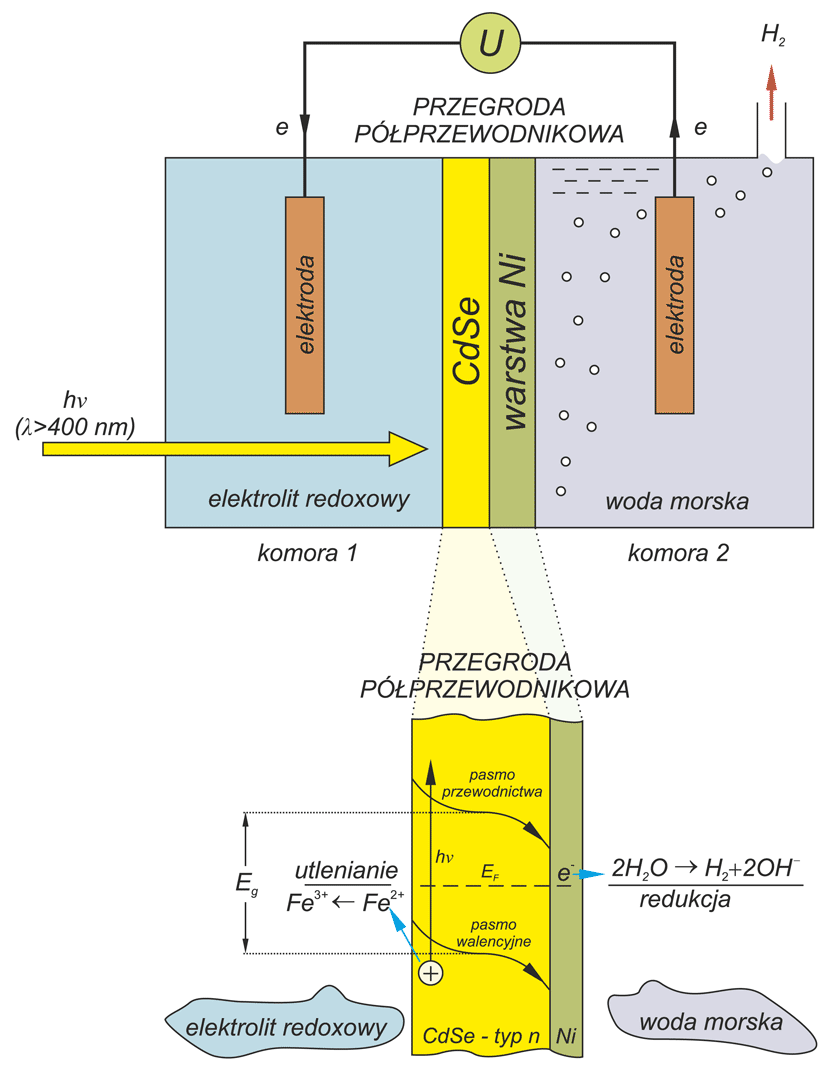
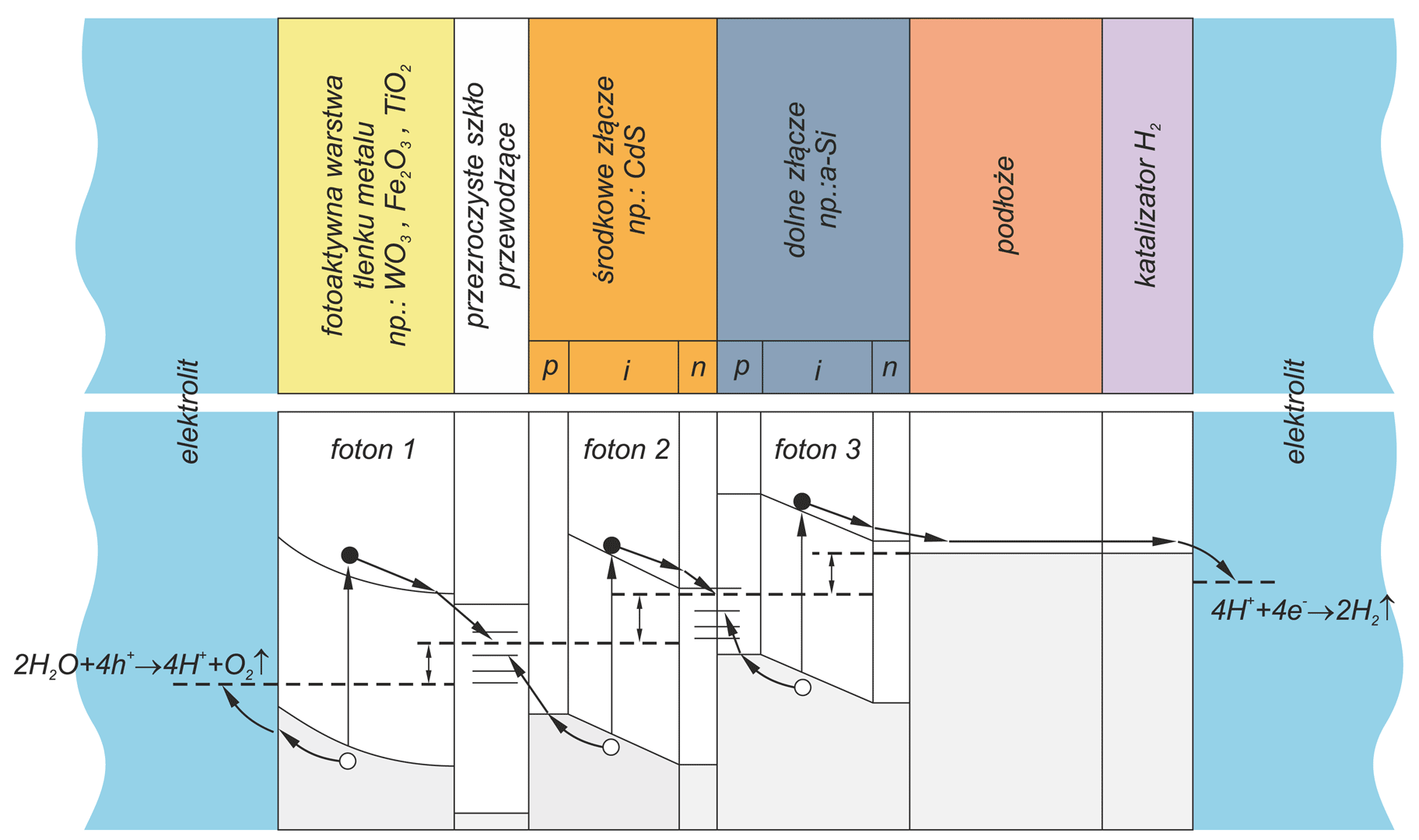
Photoelectrochemical cell for conversion solar energy
s. 6-14
DOI: 10.17274/AEZ.2015.22.01
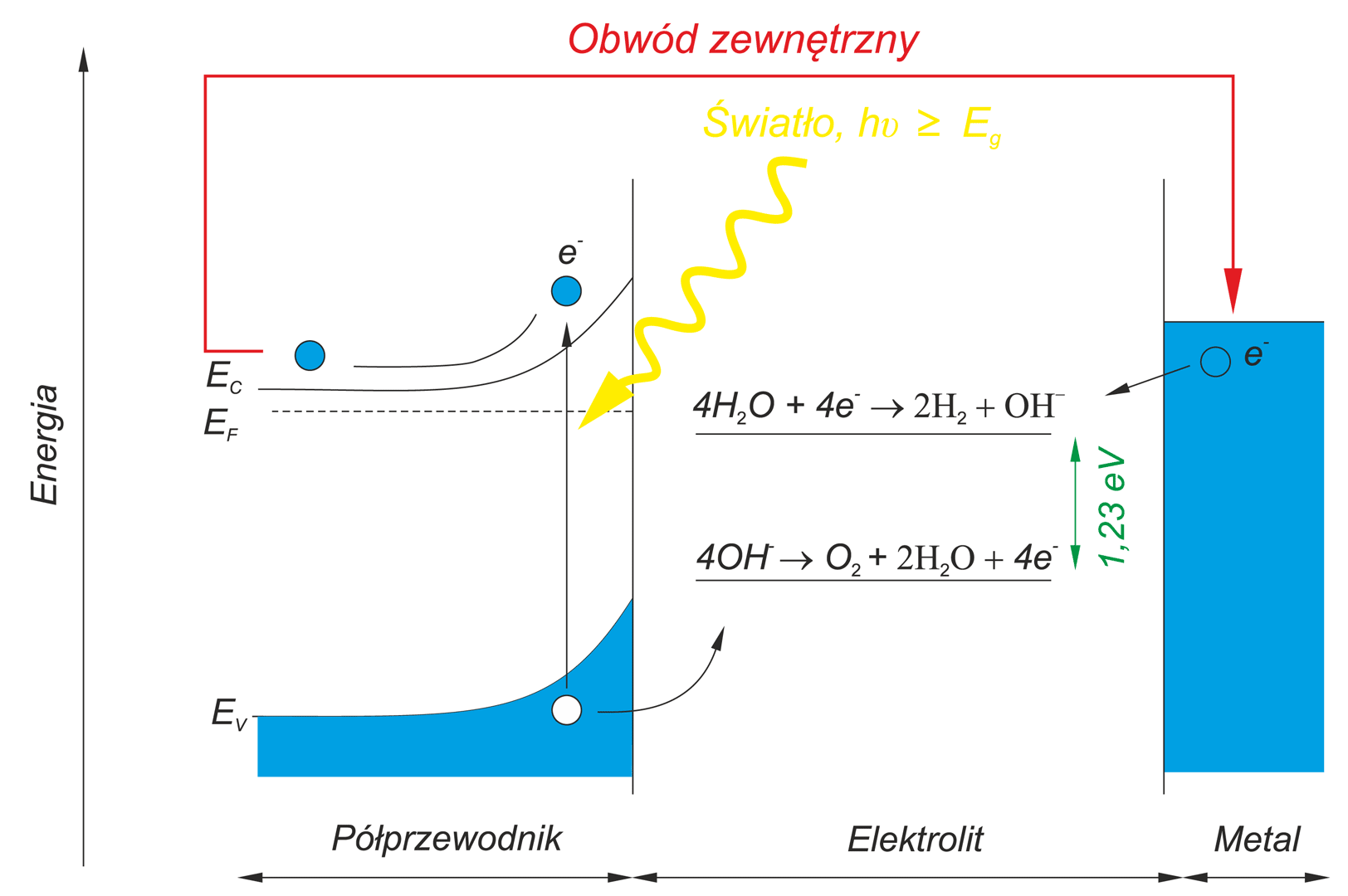
The photoelectrolysis of water – its decomposition into hydrogen and oxygen due to the absorption of light – is regarded as
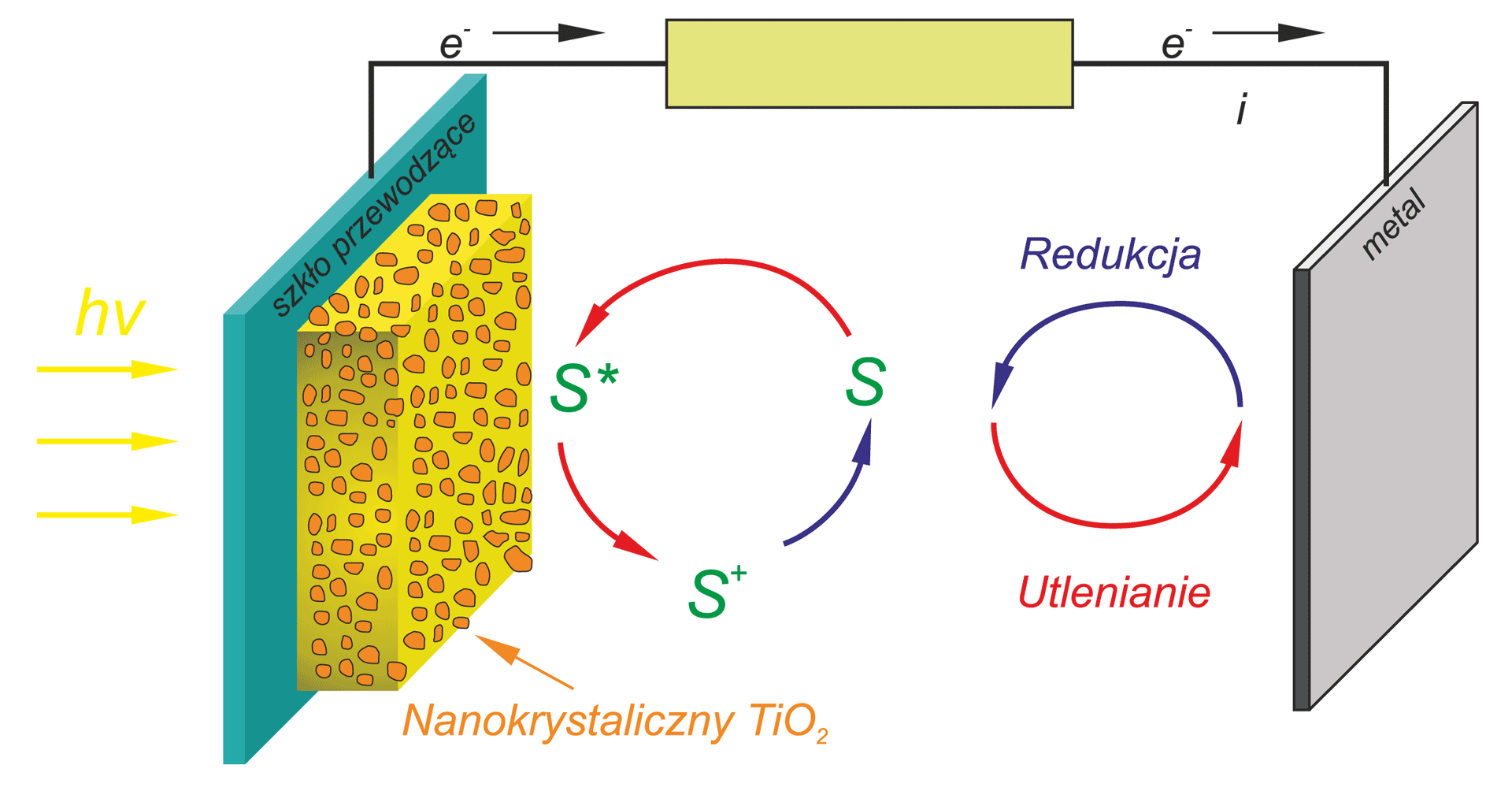
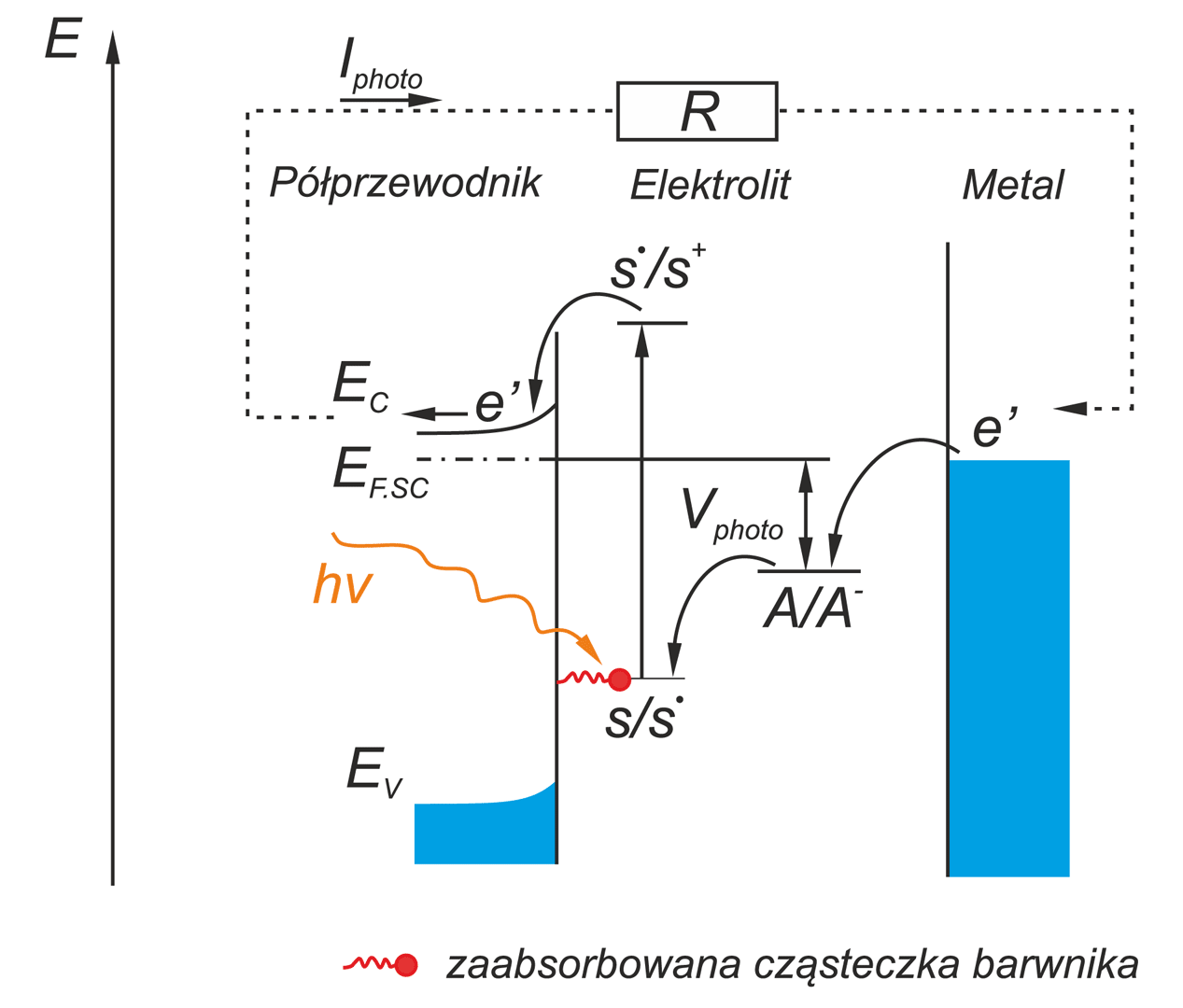
an important and non-conventional future energy source. This process proceeds in the photoelectrochemical cells, PEC, composed from
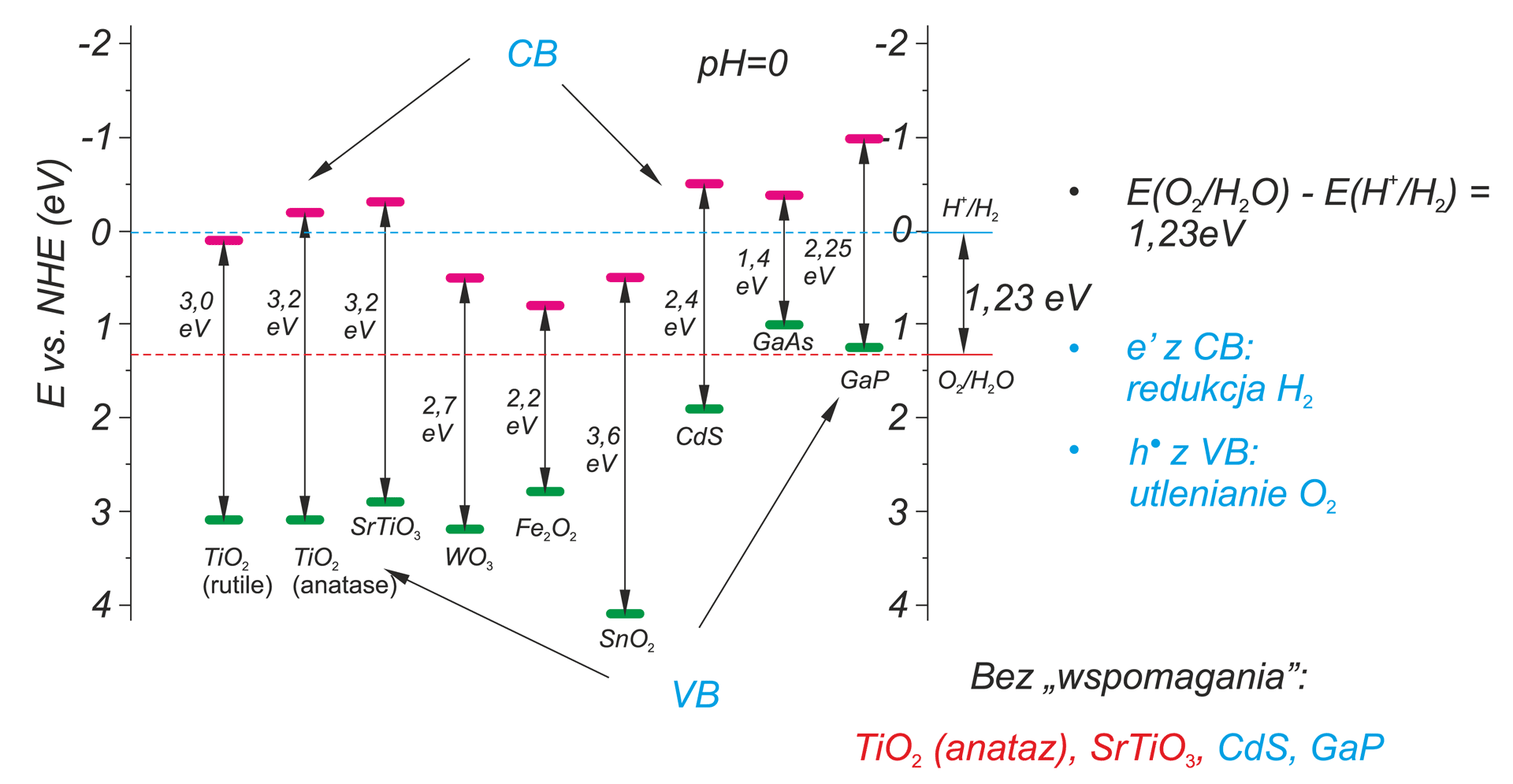
aqueous electrolyte and two electrodes. For the practical application of this process it will be necessary to find an electrode material that yields high energy conversion efficiency.
hydrogen, photoelectrochemical cells
[1] A.K Fujishima, K.Honda, “Electrochemical photolysis of water at a semiconductor electrode”, Nature, 238 (1972), 37–38
[2] M. Gratzel, “Photoelectrochemical Cell”, Nature, 414 (2001) 338–344
[3] M. Radecka, M. Rekas, K. Zakrzewska, “Titanium dioxide In photoelectrolysis of water”, Trends in Inorganic Chemistry, Review, 9 (2006)
81–125
[4] M. Radecka, M. Rękas, A. Trenczek-Zając, K. Zakrzewska, “Importance of the band gap energy and flat band potential for application
of modified TiO2 photoanodes in water photolysis”, Journal of Power Sources 181 (2008) 46–55
[5] M. Gratzel, J. Augustyński, Patent USA Nr 6936143
[6] O. Khaselev, J.A. Turner, “A monolithic photovoltaic-photoelectrochemical device for hydrogenproduction via water splitting”, Science, 280
(1998) 425–427
[7] H. Ti Tien, J.-W. Chen, “Hydrogen generation from artificial sea hater in a semiconductor septum electrochemical photovoltaic cell”,
Photochemistry and Photobiology, 49 (1999) 527–530
[8] E.L. Miller, B. Marsen, D. Paluselli, R. Rocheleau, “Optimization of hybrid photoelectrodes for solar water-splitting”, Electrochemical and Solid-
State Letters 8/5 (2005) A247–A249